UNIQUE DEVICE IDENTIFIER FAQs
On September 24, 2013, the United States Food and Drug Administration (FDA) released a final rule requiring that most medical devices distributed in the United States carry a unique device identifier (UDI). The UDI system facilitates medical device identification, traceability and tracking through distribution and use. We've prepared a selection of Frequently Asked Questions (FAQs) which follow. However, if you have other questions beyond these, please forward your questions to the UDI@terumomedical.com if you need additional information.
Frequently Asked Questions:
1. What is UDI?
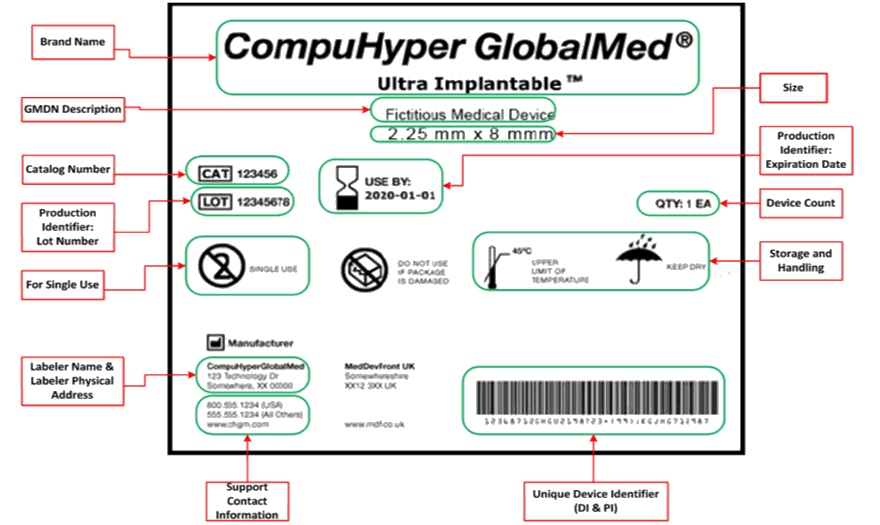
| • | A UDI is a unique numeric or alphanumeric identification code assigned to medical devices by the labeler (e.g., manufacturer) of the device. A UDI typically includes two segments: a "device identifier" (DI) and a "production identifier" (PI). | |
| • | The Global Unique Device Identification Database (GUDID), is a publicly searchable database administered by the FDA that serves as a reference catalog for every device with a UDI identifier. | |
| • | The FDA website (www.FDA.gov) has information that explains the UDI rule, the GUDID data elements and other relevant information. The following link is to the FDA's UDI website: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/UniqueDeviceIdentification/default.htm | |
| • | The FDA published a set of FAQs that may also be helpful: http://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM410439.pdf |
2. What TMC class devices are affected by this regulation?
TMC distributes Class I, II and Class III devices and is working to ensure they comply with the UDI regulation.
3. Who does the U.S. FDA UDI Rule apply to?
The requirements of the U.S. FDA UDI Rule apply to "labelers" of medical devices. The labeler of each device is responsible for meeting labeling and Global UDI Database (GUDID) data submission requirements (as well as the direct part marking and date format requirements where applicable).
4. When a customer places an order, will anything change? Will the invoice and packing slip change?
No. Customers can continue to use the Terumo product catalog numbers to place orders. The invoice and packing slip will remain the same.
5. What is the standardized date format & when does the new date standard go into effect?
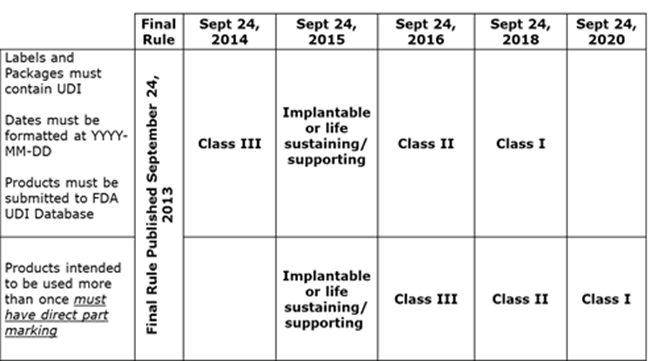
The U.S. FDA UDI Rule adopted the standard YYYY-MM-DD as the standardized format for dates on device labels. Dates on labels will have to be in the new format no later than the date on which the label of the device must bear a UDI. Consult the compliance schedule for the timelines.
6. What is the GUDID and how can data be accessed?
The GUDID serves as the repository of key device identification information, the UDI DI serves as the primary key to obtain device information in the database. The GUDID data attributes is a critical element of the FDA UDI regulation. TMC will prepare a data set specific to each DI and upload the data to the GUDID in the required timeframe. The public will be able to search information from the GUDID.
7. What about existing inventories? Do manufacturers have to remark them?
No, there are two exceptions for existing inventories:
| • | A UDI is a unique numeric or alphanumeric identification code assigned to medical devices by the labeler (e.g., manufacturer) of the device. A UDI typically includes two segments: a "device identifier" (DI) and a "production identifier" (PI). | |
| • | A device that is in commercial distribution prior to the applicable compliance date does not have to comply with the final rule. | |
| • | Devices that are manufactured and labeled before their compliance date have an exception from the rule. However, this exception expires 3 years after the compliance date for that device. |
8. Do all devices need to be directly marked with their UDI?
No. The rule only requires direct part marking for re-usable medical devices that need to be reprocessed before reuse.
9. Will UDI drive any changes with the barcodes on product labels?
The FDA stated in the final FDA rule: "FDA does not require the use of specific forms of AIDC or specific AIDC technologies." The Companies of Terumo Medical Corp will select the GS1 bar code symbol (Terumo's selected UDI System) appropriate to the size of the package and the scanning environment (for instance, bedside scanning versus scanning in a warehouse). 2D Datamatrix symbols may be used on small packages. Larger packages may use GS1-128 linear bar codes.
10. What are key FDA regulatory milestones for UDI?
The FDA has established a specific timetable by which medical devices must be compliant. Class III devices are the first group of devices that must comply, followed by Class II and Class I. If these dates are not met, the product cannot be distributed in the U.S. The FDA has also granted an existing inventory exception of 3 years for a finished device manufactured and labeled prior to the UDI compliance date.