Terumo Announces European Launch of Biosimilar Using Terumo’s PLAJEX™ Pre-fillable Syringes

TOKYO, JAPAN – December 7, 2018 – Terumo Corporation (TSE: 4543) today announced that a biosimilar, which uses the company’s PLAJEX™ pre-fillable syringe, has launched in Europe. Terumo manufactures the product Hulio®, a biosimilar to Humira® (adalimumab), on contract. This marks an important milestone, as the first overseas commercial launch for PLAJEX™.
Fujifilm Kyowa Kirin Biologics Co., Ltd. and Terumo jointly designed the formulation of Hulio®. Terumo Yamaguchi D&D Corporation, a wholly-owned subsidiary of Terumo Corporation, is conducting the manufacturing on contract, and Mylan N.V. will commercialize the product in Europe. Hulio® is available in two forms: a pre-filled auto-injector suited to self-injection; and a pre-filled syringe whose design especially focuses on medical safety. *
“Patient safety is our first priority, and PLAJEX™, which is a polymer pre-fillable syringe specially developed for use with biopharmaceuticals, matched our needs seamlessly for the use in Hulio®. The polymer material is harder to break and safer to use compared to the conventional glass syringe,” commented Dr. Yoshifumi Torii, President and CEO of Fujifilm Kyowa Kirin Biologics Co., Ltd.
“The design and manufacturing method of PLAJEX™ is also unique, being silicone oil-free, and using autoclave sterilization. This contributes to reduce risks such as aggregation and oxidation of the biopharmaceuticals. We are confident that this exciting collaboration will help elevate patients’ QOL,” commented Tetsuya Kumei, Division President of the Alliance Division, General Hospital Company, Terumo Corporation.
Going forward, Terumo will use its material manufacturing technologies and filling technology for pre-fillable syringes to continue expanding its alliance businesses with pharmaceutical companies.
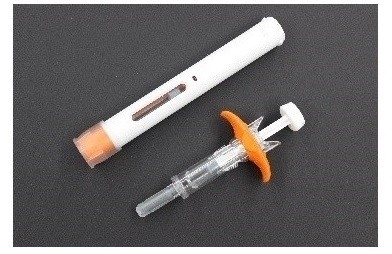 auto-injector (above) and pre-filled syringe (below)
auto-injector (above) and pre-filled syringe (below)
*The auto-injection pen incorporates Switzerland-based Ypsomed’s auto-injector, and the pre-filled syringe incorporates France-based Nemera’s safety device.
About Terumo Corporation
Tokyo-based Terumo Corporation is one of the world's leading medical device manufacturers, with over $5 billion in sales and operations in more than 160 nations. Founded in 1921, the company develops, manufactures and distributes world-class medical devices, including products for use in cardiothoracic surgery, interventional procedures and transfusion medicine; the company also manufactures a broad array of syringe and hypodermic needle products for hospital and physician-office use. Terumo contributes to society by providing valued products and services to the health care market, and by responding to the needs of health care providers and the people they serve. Terumo Corporation's shares are listed on the first section of the Tokyo Stock Exchange (No. 4543, Reuters symbol <4543.T>, or Bloomberg 4543: JP) and is a component of the Nikkei 225, Japan's leading stock index.
###





