Terumo’s PLAJEX Enters US Markets by Receiving GMP Certification from FDA to Manufacture Biosimilar Hulio
TOKYO, JAPAN – August 3, 2020 – Terumo Corporation (TSE: 4543) announced that its affiliate company Terumo Yamaguchi D&D Corporation has received Good Manufacturing Practice (GMP) certification from the US Food and Drug Administration (FDA), in regards to a biosimilar product Terumo will manufacture on contract.
The GMP certification ensures the manufacturing processes and quality management systems at the manufacturing facility meet adequate regulation standards. Terumo Yamaguchi D&D will manufacture Hulio® (adalimumab-fkjp), a biosimilar to AbbVie’s Humira® (adalimumab). The drug is developed and owned by Fujifilm Kyowa Kirin Biologics Co., Ltd and it has received FDA approval this July.
Hulio will be used for the treatment of diseases such as rheumatoid arthritis and psoriatic arthritis. Fujifilm Kyowa Kirin Biologics tied partnership with Mylan N.V., for the commercialization of Hulio in the European markets in 2018. In June 2020, the product also received approval for commercialization in Japan. Followed by the latest US approval, Mylan will prepare for launch of Hulio in the US during 2023.
Hulio is filled in Terumo’s pre-fillable polymer syringe PLAJEX™. Biopharmaceuticals are composed of proteins as active ingredients; therefore, the drug is highly sensitive to impurities, temperature, light and other external factors. The unique material used in PLAJEX is expected to reduce drug absorption risks and impurity dissolution. Tetsuya Kumei, Division President, the Alliance Division, General Hospital Company of Terumo comments, “PLAJEX is a one of a kind syringe, and is used in various products available on the market today. However, we are excited to announce for the first time that a product filled in PLAJEX has been approved for the US market”.
Terumo will continue to build strong alliances with pharmaceutical companies and contribute to better healthcare.
 Manufacturing line of PLAJEX
Manufacturing line of PLAJEX
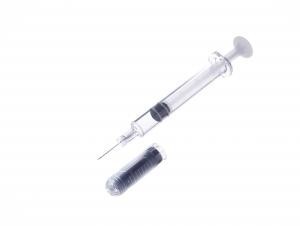 Pre-fillable polymer syringe PLAJEX
Pre-fillable polymer syringe PLAJEX
For Reference
Terumo “Our Technology” - #03 Technology that Meets Patients’ Medication Needs with Safety and Certainty
https://www.terumo.com/technology/stories/03/
About Terumo
Terumo (TSE:4543) is a global leader in medical technology and has been committed to "Contributing to Society through Healthcare" for nearly 100 years. Based in Tokyo and operating globally, Terumo employs more than 25,000 associates worldwide to provide innovative medical solutions in more than 160 countries and regions. The company started as a Japanese thermometer manufacturer, and has been supporting healthcare ever since. Now, its extensive business portfolio ranges from vascular intervention and cardio-surgical solutions, blood transfusion and cell therapy technology, to medical products essential for daily clinical practice. Terumo will further strive to be of value to patients, medical professionals, and society at large.
###





